Clinical Research Tracker
Clinical Studies Exploring the Use of Cell-Based Therapies for Patients in Need
Currently, 1,053 clinical studies are listed on www.clinicaltrials.gov that explore the use of cell-based therapies for patients with various conditions.
Clinical Studies Exploring Use of Cell-Based Therapies by Condition (n=1,053)
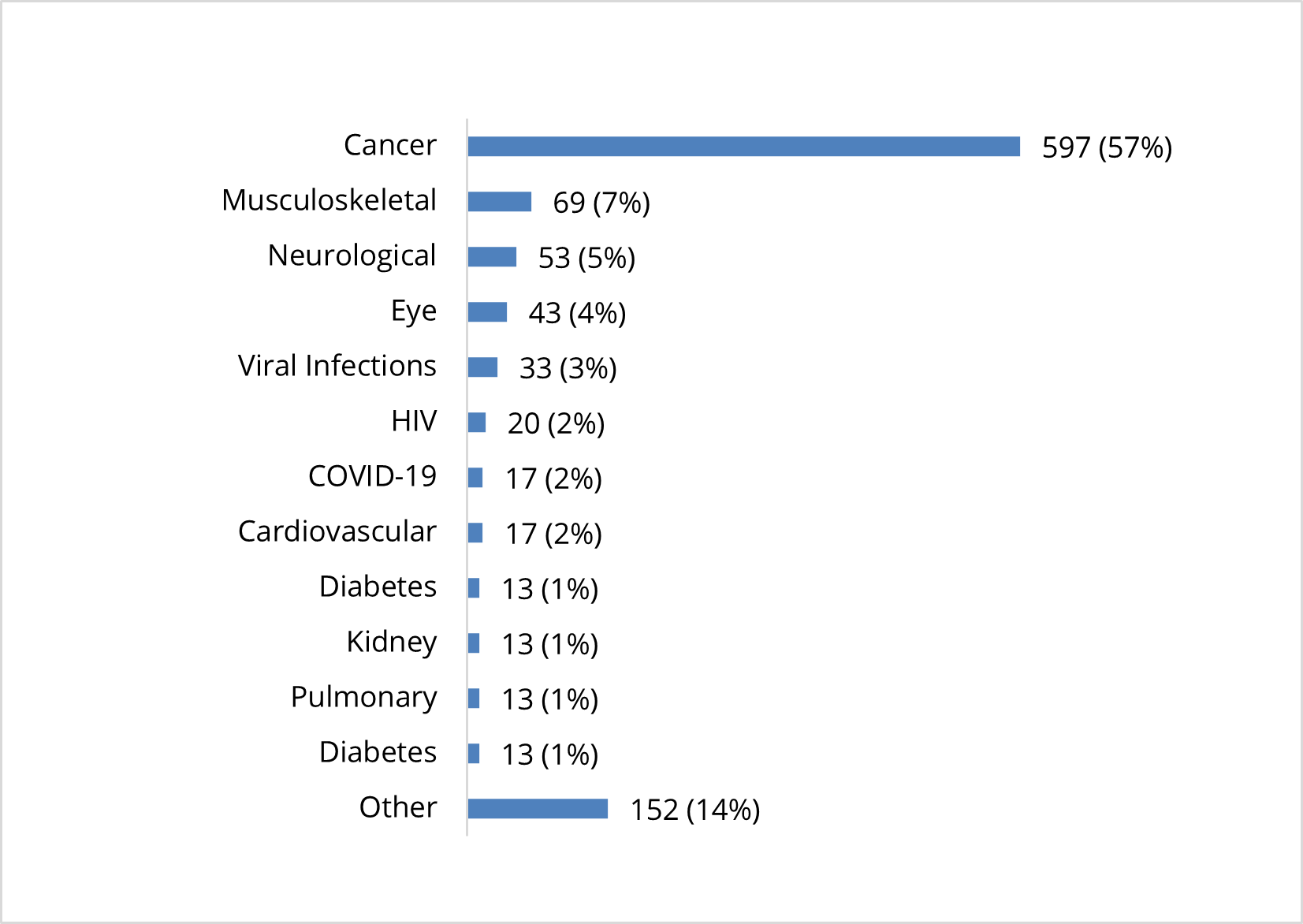
There are more than 1,000 active U.S. clinical studies that were exploring the use of cell-based therapies for patients in need. Cancer is the most prevalent condition being studied, representing 57% of all active clinical studies, followed by musculoskeletal conditions (7%), neurological conditions (5%), and eye-related conditions (4%). Studies are also focusing on cardiovascular disease, COVID-19, diabetes, HIV, kidney disease, pulmonary conditions, viral infections and other conditions.
Clinical Studies Exploring Use of Cell-Based Therapies by General Product Type (n=1,053)
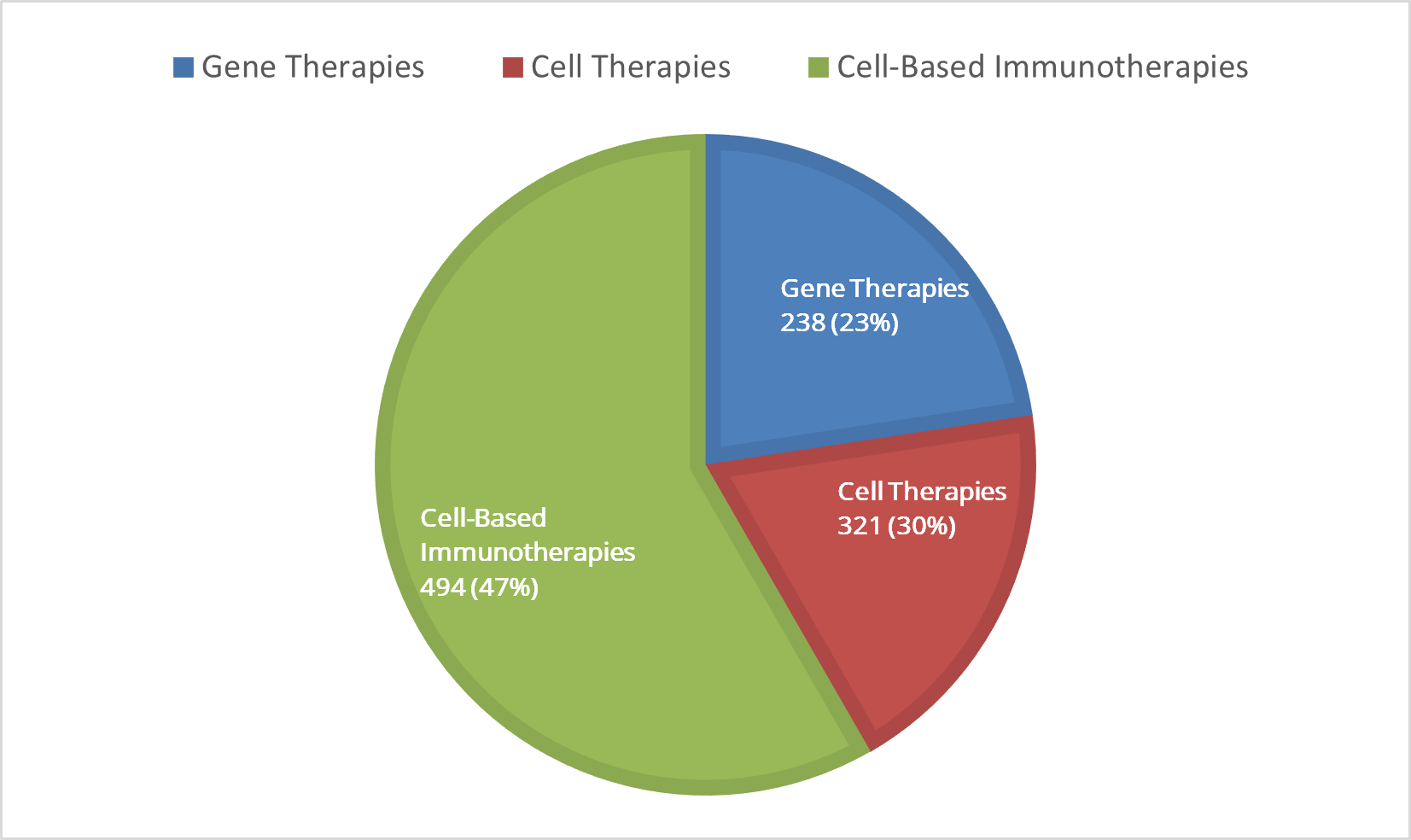
Forty-seven percent of active U.S. clinical studies are exploring the use of cell-based immunotherapies, while 30% are exploring the use of cell-based therapies, followed by gene therapies at 23%.
Clinical Studies Exploring Use of Cell-Based Therapies by Specific Product Type (n=1,053)
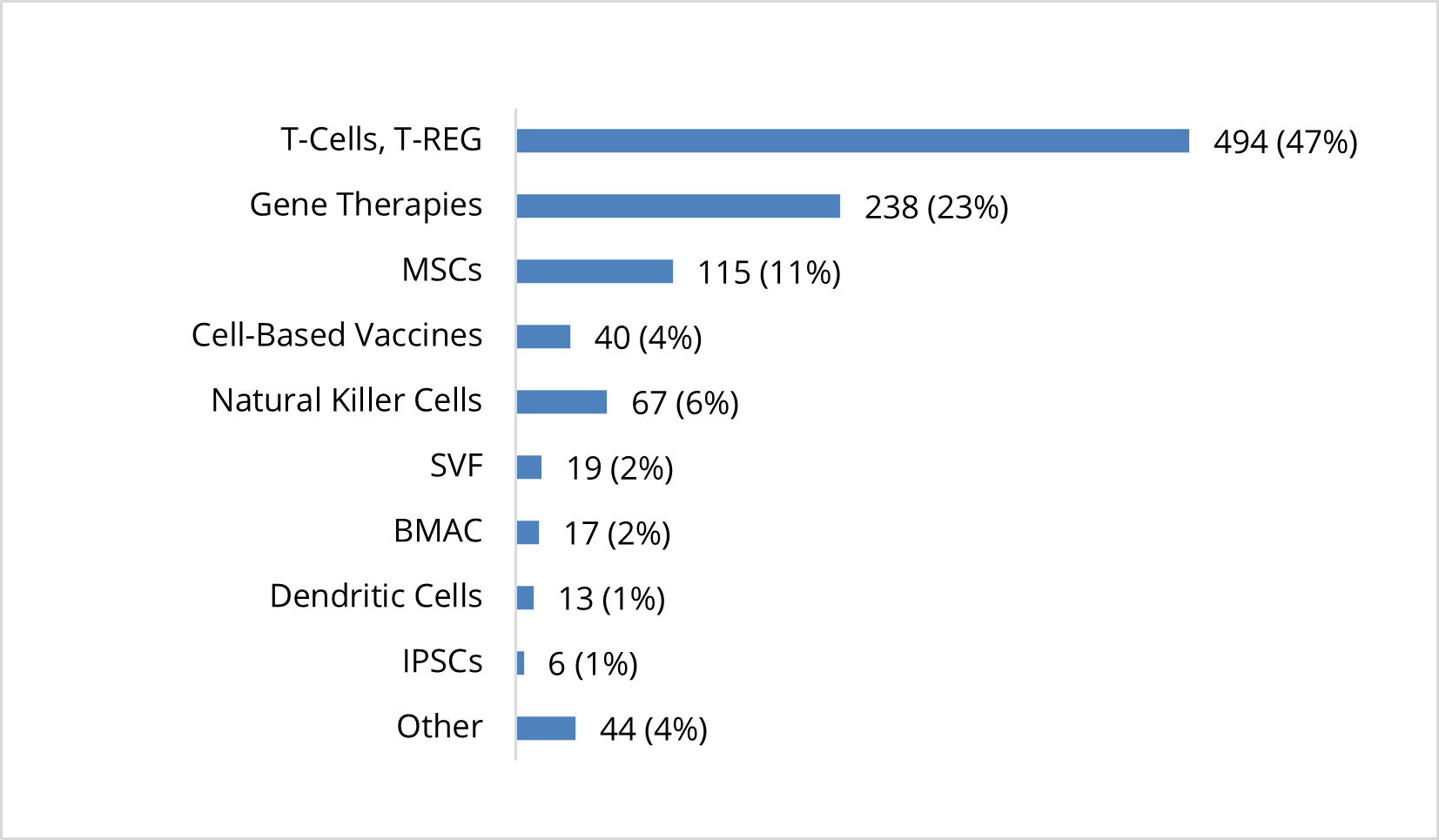
T-cells and T-Reg cells make up the majority (or 80%) of the 614 cell-based immunotherapies being explored, while 67 (or 11%) are natural killer cells. Most of the 201 active clinical studies related to cell-based therapies are focused on MSCs (115 or 57%), followed by stromal vascular fraction (SVF) (19 or 9%), bone marrow aspirate concentrate (17 or 8%), induced pluripotent stem cells (iPSCs) (6 or 3%), and other cell therapies (44 or 22%).
Clinical Trials Exploring the Use of Cell-Based Therapies By Phase (n=954)
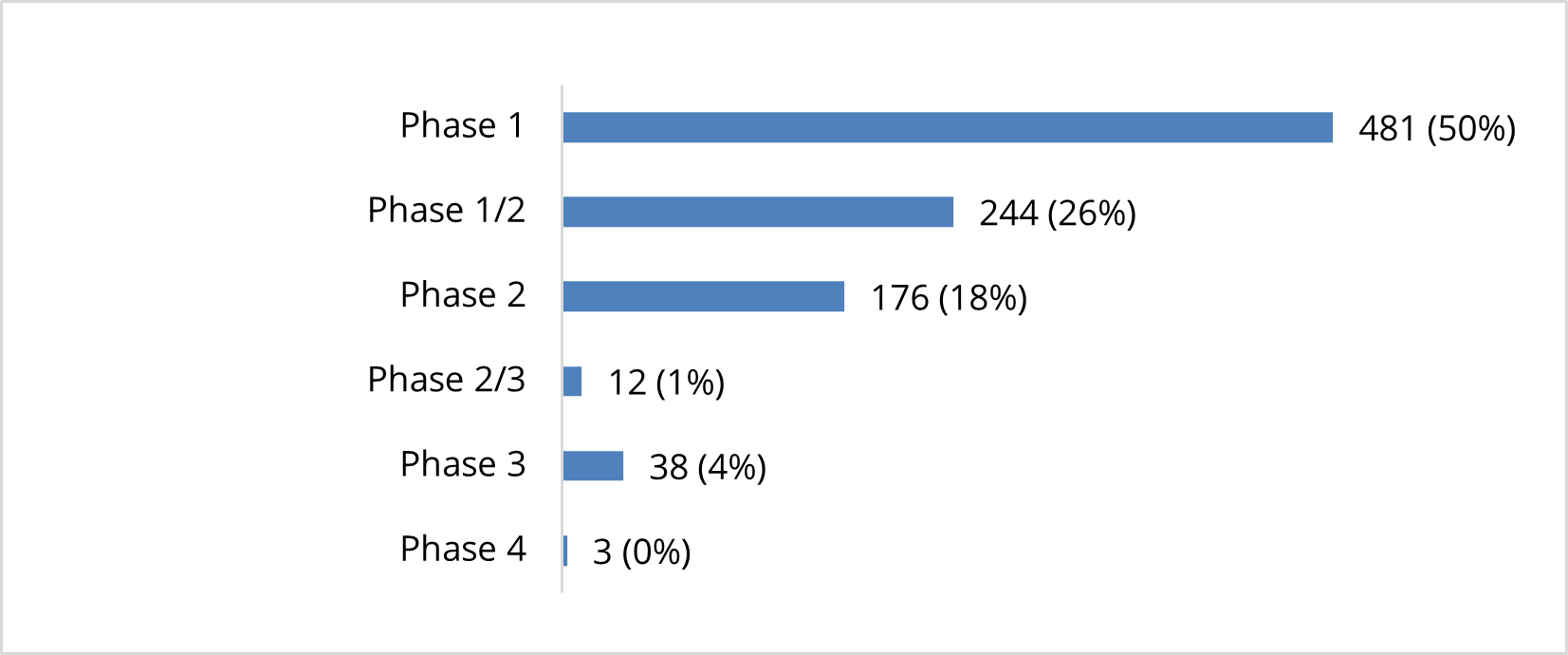
The vast majority of clinical trials (94%) are either Phase 1 or Phase 2 studies. Fifty percent are Phase 1 studies, 26% are Phase 1/2 studies, and 18% are Phase 2 studies. One percent of active clinical trials are Phase 2/3 studies, while 4% are Phase 3 studies.
U.S. Clinical Studies Exploring Use of Cell-Based Therapies By State (n=1,053)
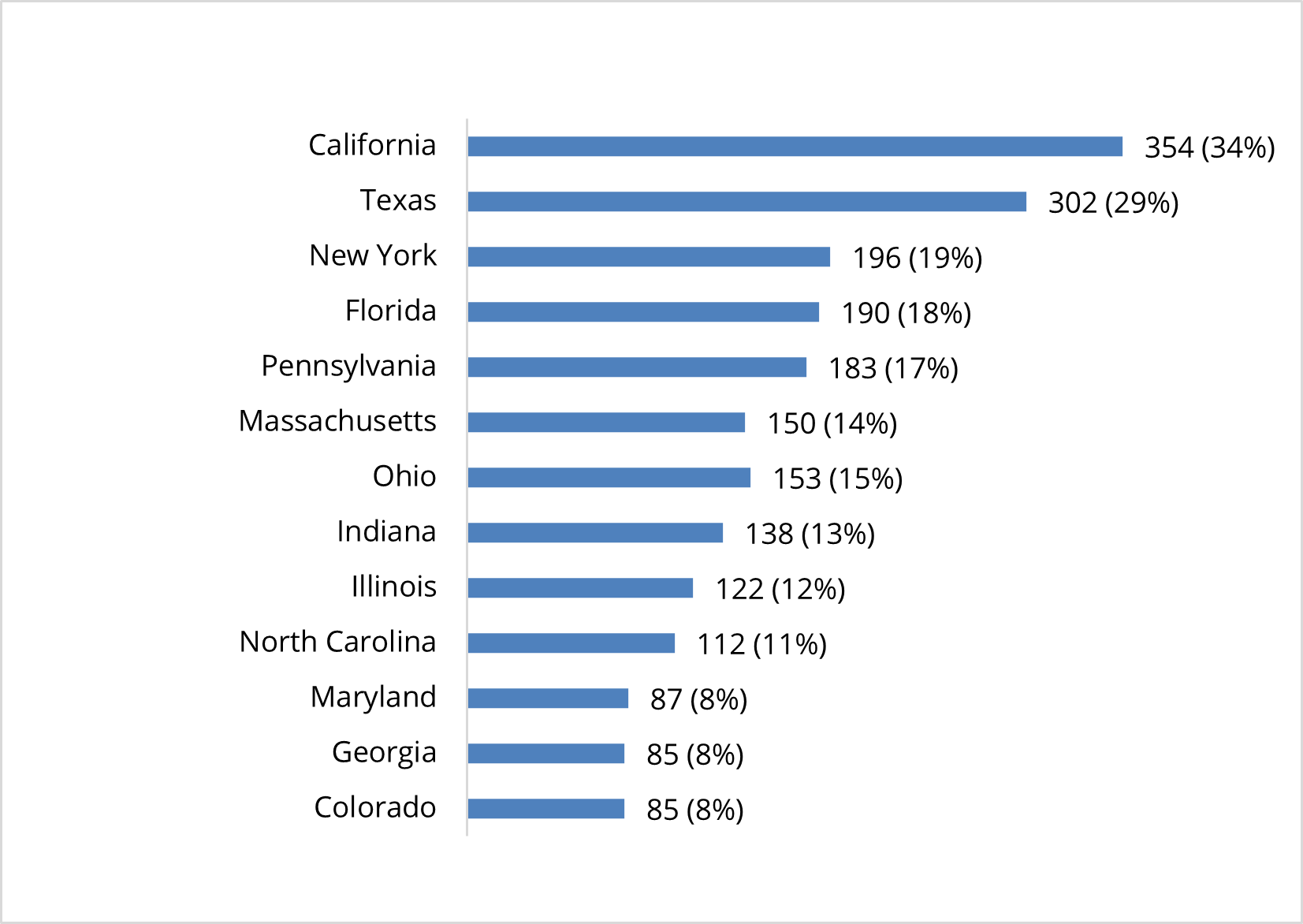
Thirty-four percent of active clinical studies are being conducted in California, 29% are being conducted in Texas, 19% are being conducted in New York, 18% are being conducted in Florida, and 17% are being conducted in Pennsylvania. A number of clinical studies are also being conducted in Massachusetts, Ohio, Indiana, Illinois, and North Carolina, as well as Maryland, Georgia, and Colorado.
Sources of Funding for Active U.S. Clinical Studies Exploring Use of Cell-Based Therapies (n=1,053)
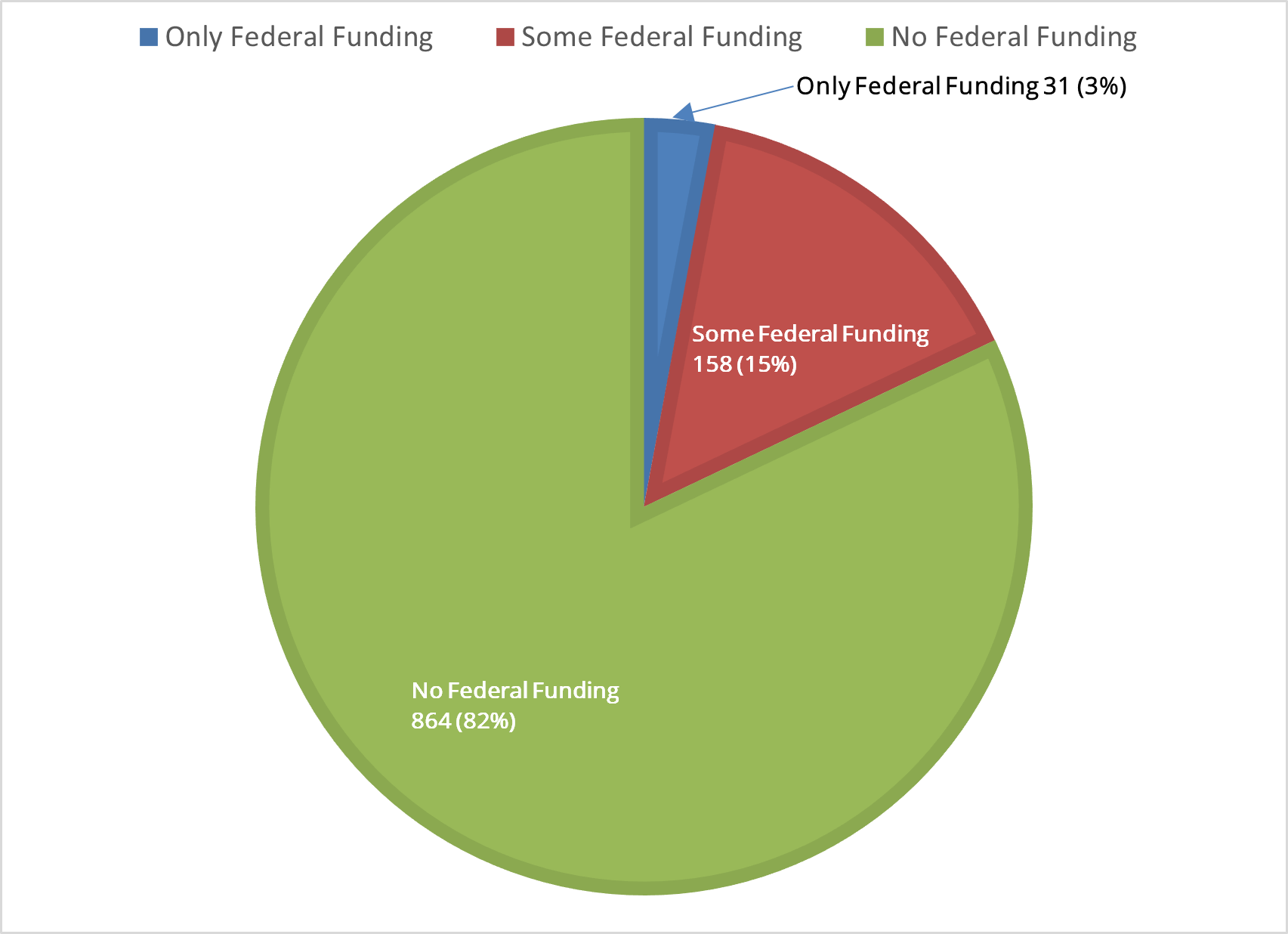
Only 18% of active U.S. clinical studies have any federal support. Fifteen percent have some federal support and 2% are solely funded by the federal government. The remaining 82% of clinical studies are funded by sources outside of government.