COVID-19 Clinical Research Tracker
Clinical Studies Exploring Use of Cell-Based Therapies for COVID-19
Currently, 130 clinical studies are listed on www.clinicaltrials.gov that explore the use of cell-based therapies for patients with COVID-19.
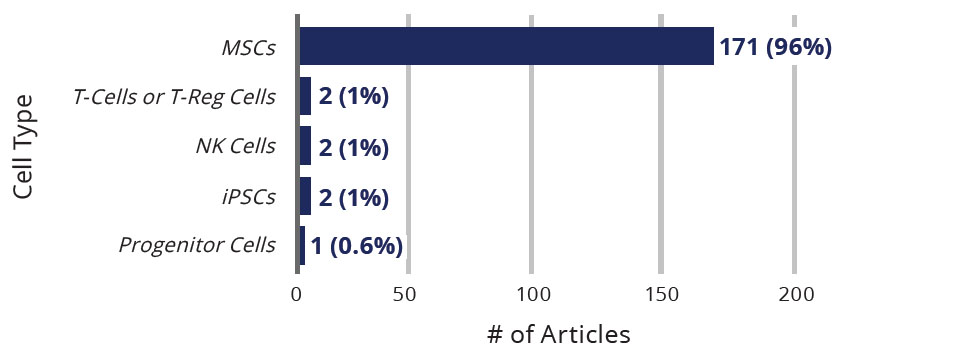
At least 178 review articles have been published in peer-reviewed publications offering insights on the potential or promise of cell-based therapies for patients with COVID-19. The vast majority (171 or 96%) of such review articles describe the role of MSCs in treating COVID-19 patients.
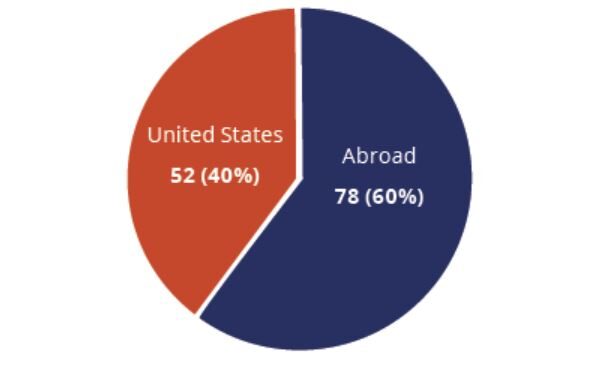
Clinical Studies Exploring Use Of Cell-Based Therapies for COVID-19 By Location (n=130)
All Clinical Studies Exploring Use of Cell Based Therapies For COVID-19 By Phase (n=130)
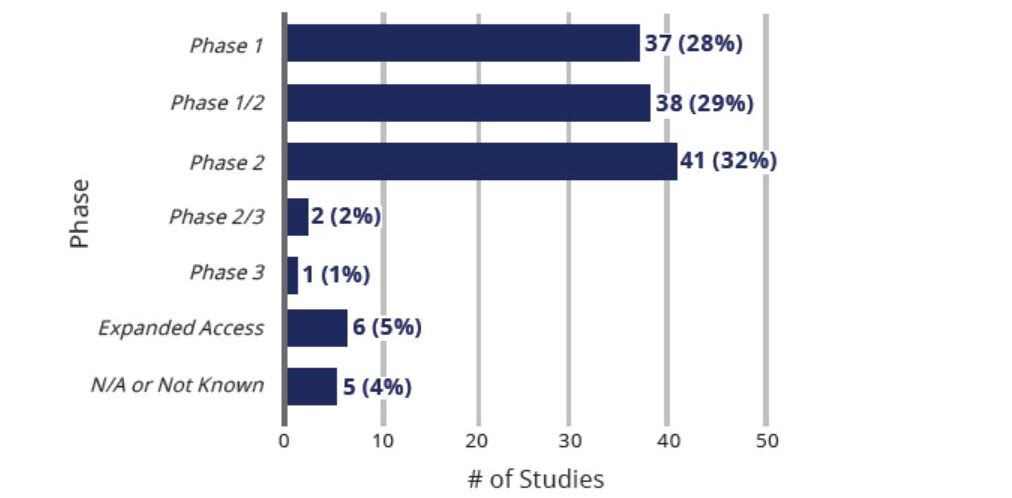
The vast majority of studies (89%) are either Phase 1 (28%), Phase 2 (29%), or Phase 1/2 (32%) clinical trials.
Two of the studies are Phase 2/3 clinical trials and only one is a Phase 3 clinical trial.
There are 6 expanded access studies and the phase is not known or applicable for the remaining 5 studies.
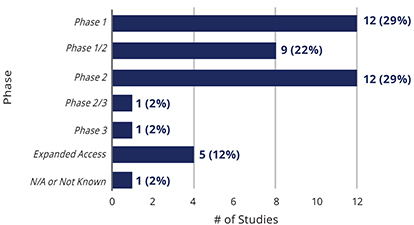
U.S. Clinical Studies Exploring Use of Cell-Based Therapies for COVID-19 By Phase (n=41)
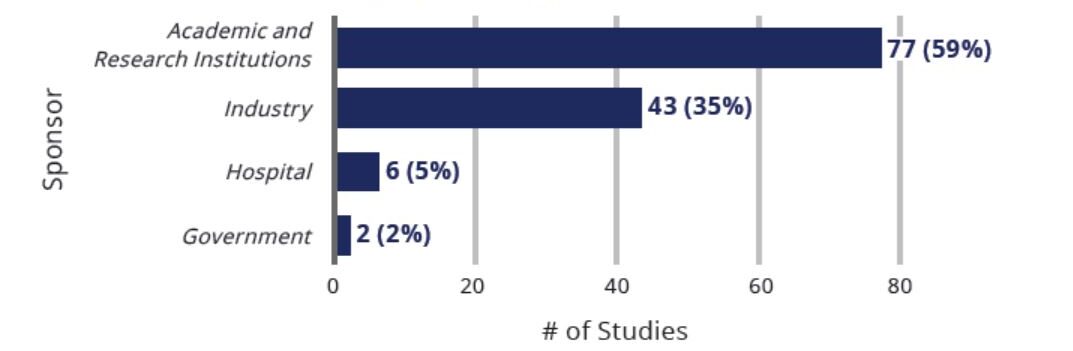
Clinical Studies Exploring Use Of Cell-Based Therapies for COVID-19 By Sponsor Type (n=130)
Clinical Studies Exploring Use Of Cell-Based Therapies for COVID-19 By Cell Type (n=130)
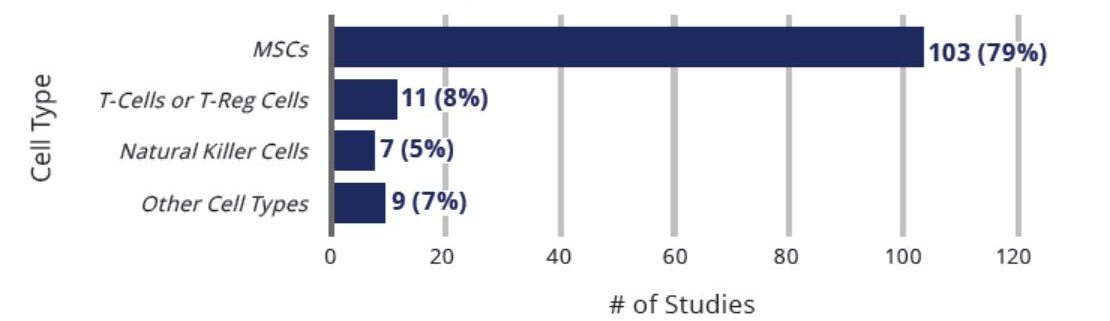
The vast majority (103 or 79%) of the 130 studies conducted globally are focused on the use of MSCs for patients with COVID-19. Other cell-based therapies are being explored, including T-cells and T-Reg cells, NK cells, and other cells.
Published Review Articles Exploring Use of Cell-Based Therapies for COVID-19
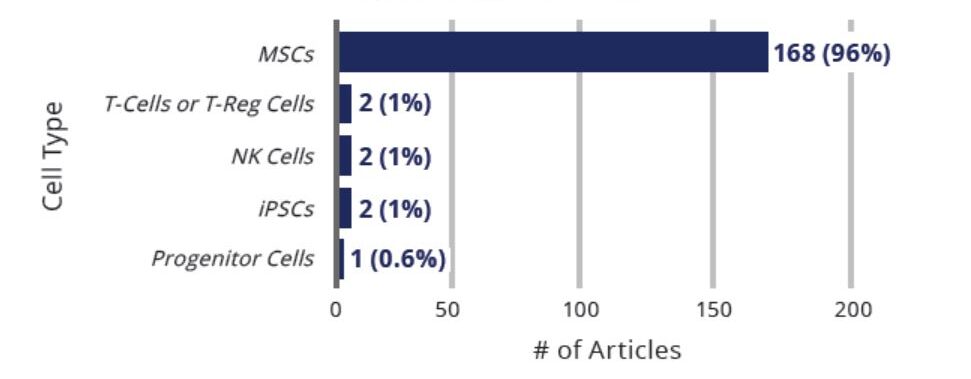
At least 175 articles have been published in peer-reviewed publications that offer insights on the potential or promise of regenerative medicine and cell therapies for patients with COVID-19. See below for a summary of the number of articles by cell type.
The vast majority (96%) of published articles describe the role of MSCs in treating patients with COVID-19. The remaining articles explore the potential of T-reg cells (2), iPSCs (2), natural killer (NK) cells (1), and progenitor cells (1).