COVID-19 Clinical Research Tracker
Clinical Studies Exploring Use of RMCTs for COVID-19
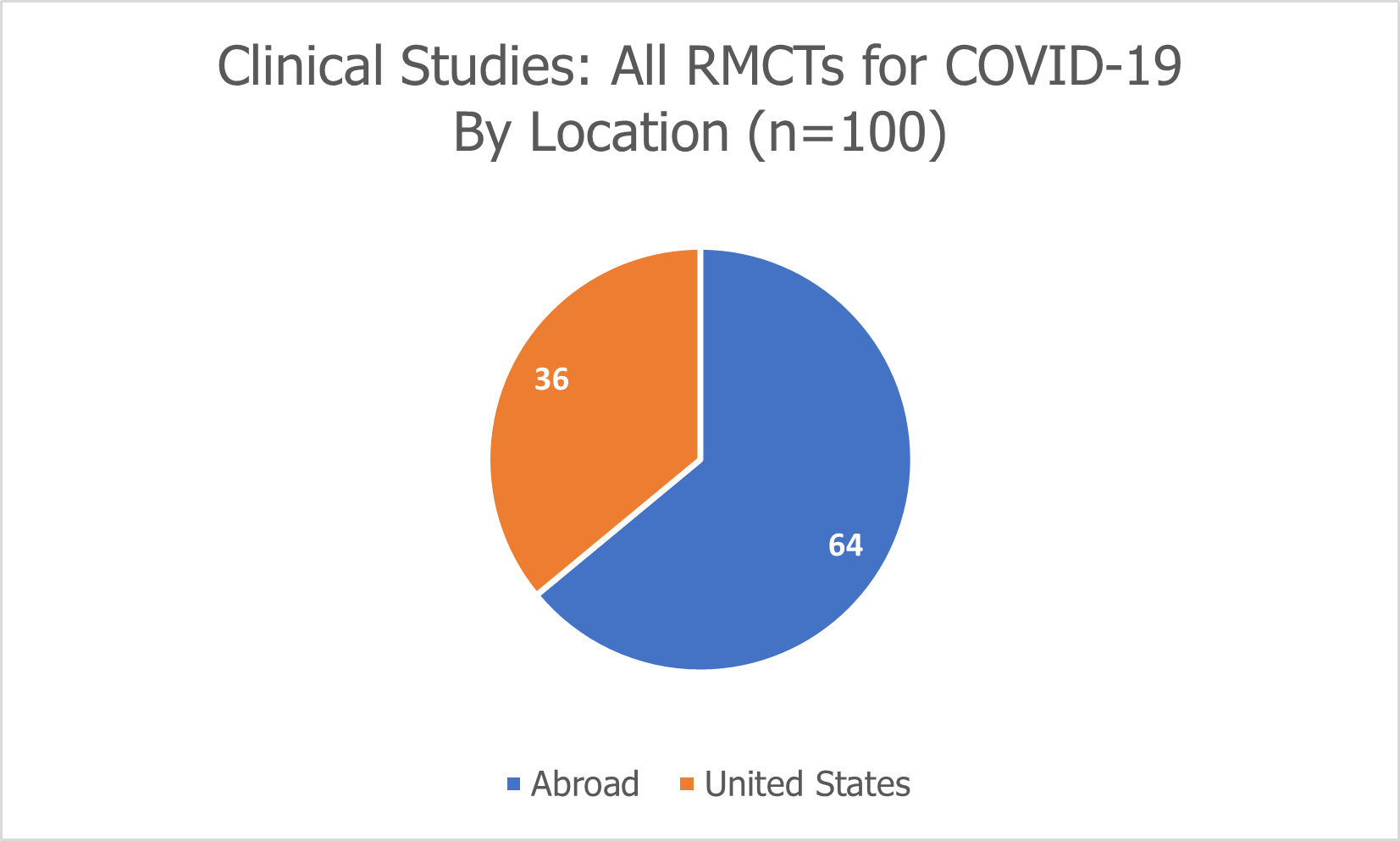
Currently, 100 clinical studies are listed on www.clinicaltrials.gov that explore the use of regenerative medicine and cell therapies (RMCTs) for patients with COVID-19.
36 of the 100 clinical studies are being conducted in the United States, while 64 are being conducted abroad.
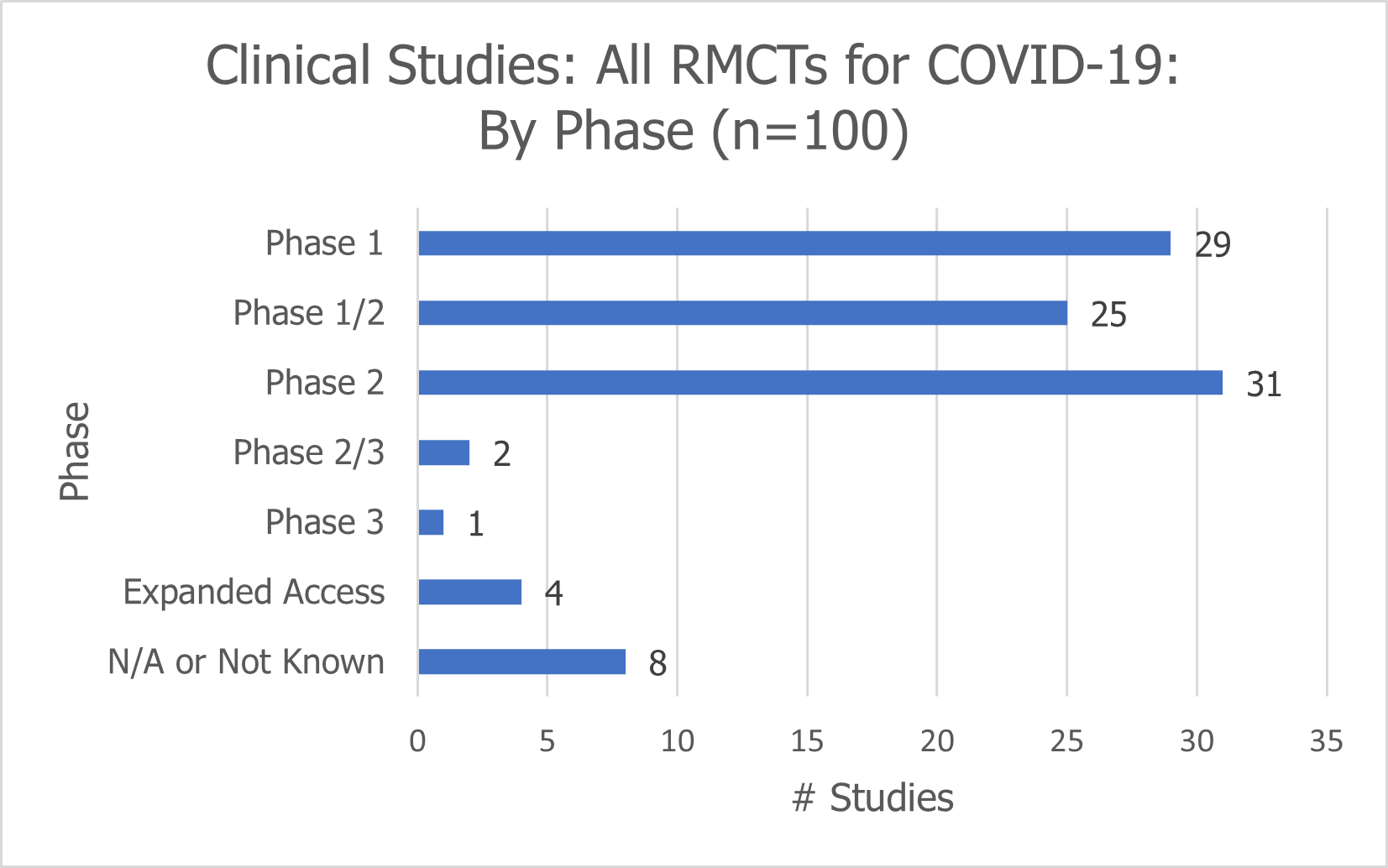
The vast majority of studies (85%) are either Phase 1 (29), Phase 2 (31), or Phase 1/2 (25) clinical trials.
Two of the studies are Phase 2/3 clinical trials and only one is a Phase 3 clinical trial.
There are 4 expanded access studies; the phase is not known or applicable for the remaining 8 studies.
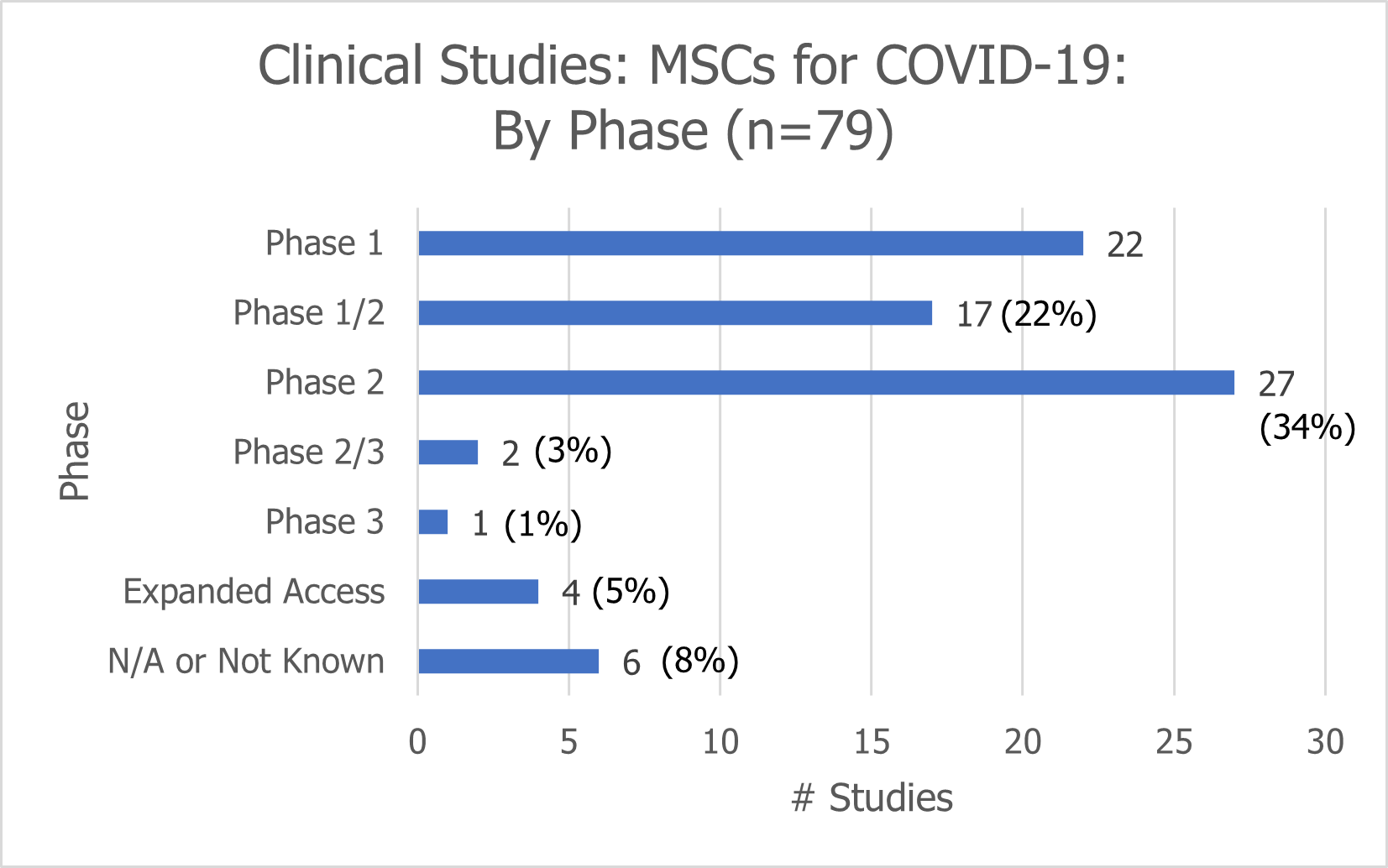
Of the 79 clinical studies exploring the use of MSCs for COVID-19, 27 are being conducted in the United States.
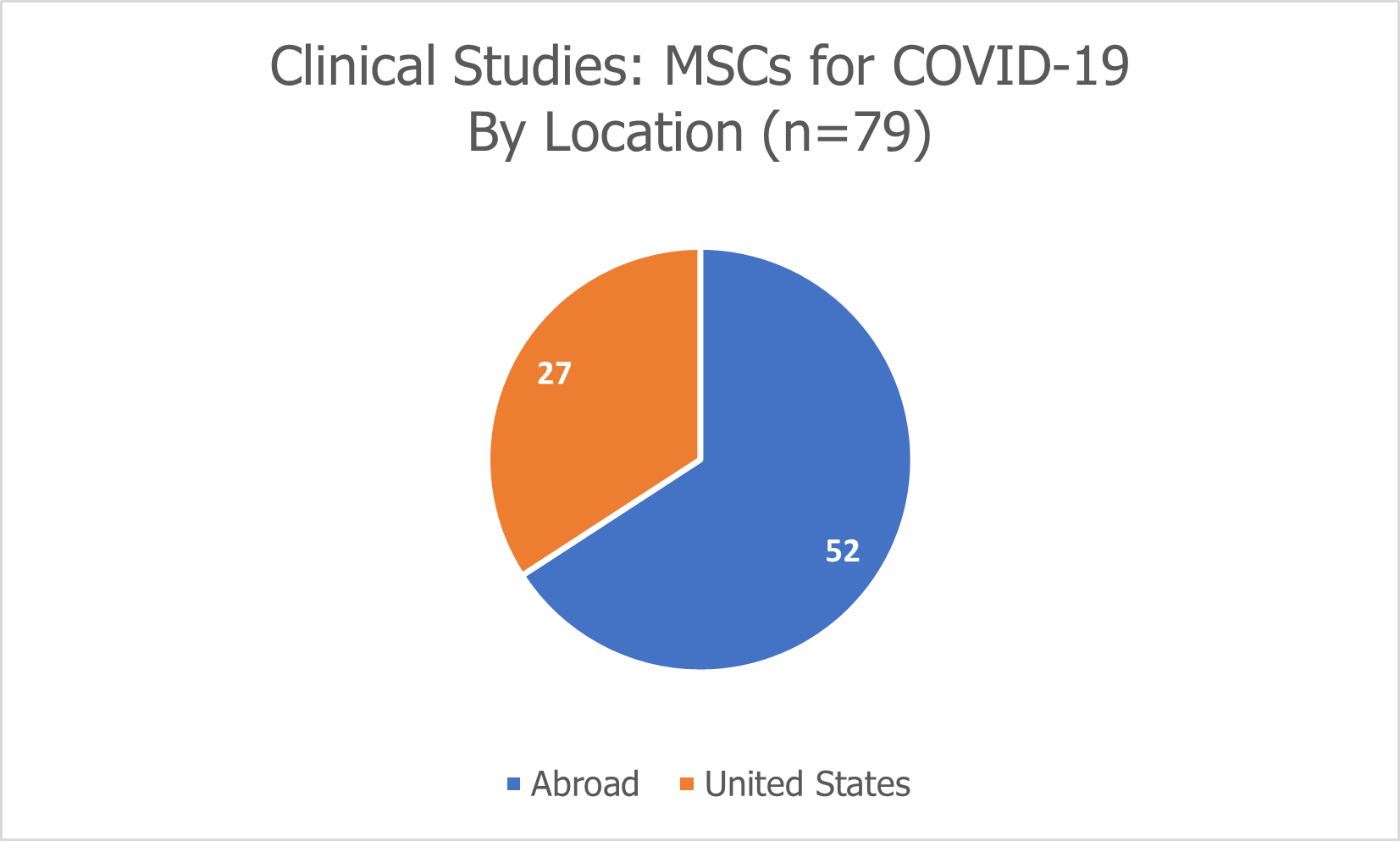
79 of the 100 studies involve the use of mesenchymal stromal cells (MSCs).
-
27 (or 34%) are being conducted in the U.S.
-
66 (or 84%) are Phase 1 (28%), Phase 2 (34%), or Phase 1/2 (23%) clinical trials
-
Two studies (3%) are Phase 2/3 clinical trials and only one study is a Phase 3 clinical trial
Only one of the studies–a Phase 1 trial–has received federal funding (through the National Cancer Institute).
Published Review Articles Exploring Use of RMCTs for COVID-19
Several articles have been published in peer-reviewed publications that offer insights on the potential or promise of regenerative medicine and cell therapies for patients with COVID-19. Click here for a list of the articles. See below for a summary of the number of articles by cell type.
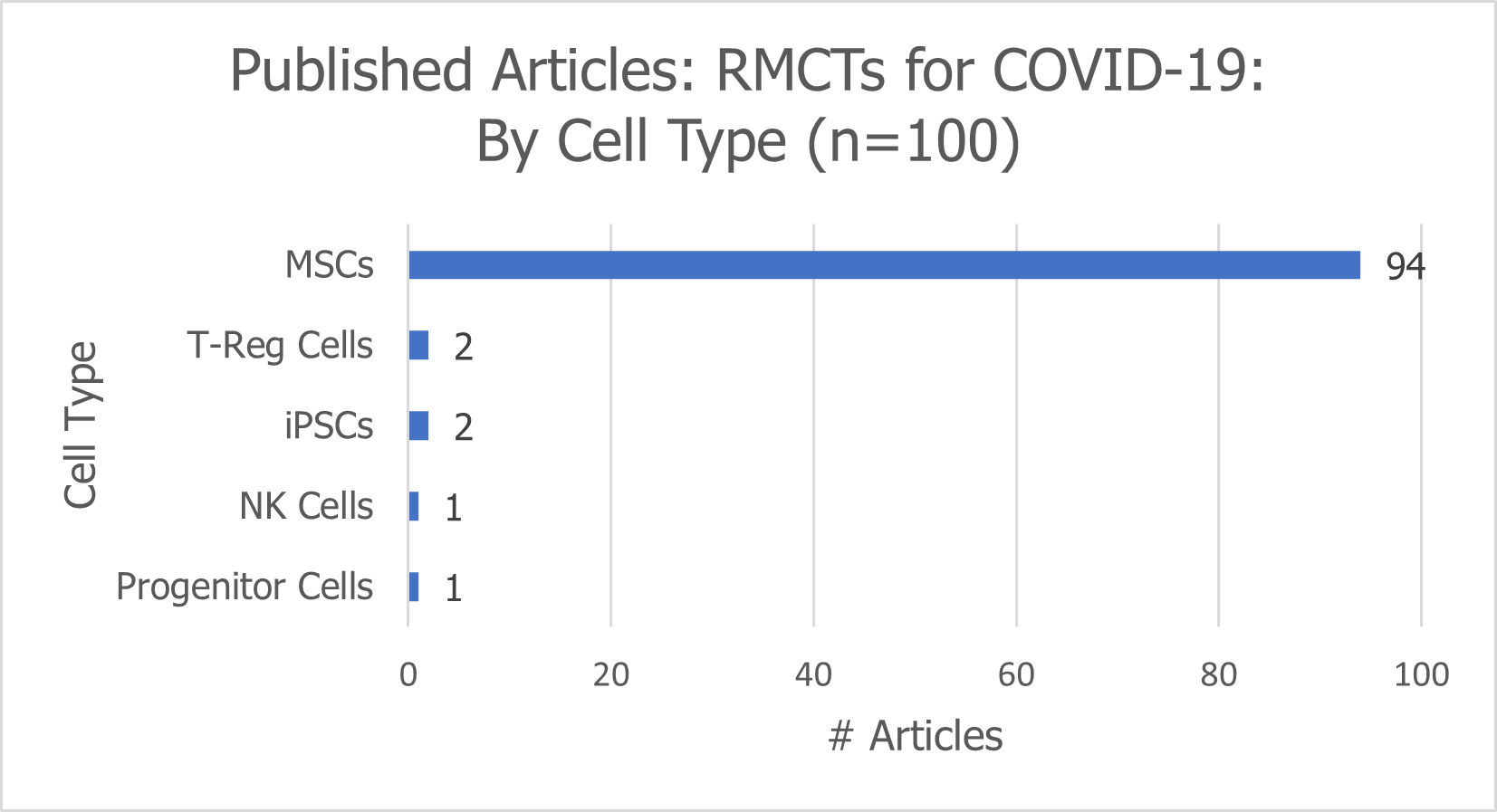
There are 100 published articles that describe the promise or potential of RMCTs for patients with COVID-19. The majority (94%) describe the promise or potential of MSCs.
The remaining articles explore the potential of T-reg cells (2), iPSCs (2), NK cells (1), and progenitor cells (1) in treating patients with COVID-19.
Published Results from Clinical Studies Exploring Use of RMCTs for COVID-19
Researchers involved in 21 clinical studies involving the use of RMCTS for severely ill patients with COVID-19 have published their results, 19 of which were published or are to be published in peer-reviewed journals.
- Across all 19 studies with results published in peer-reviewed journals, mortality rates averaged 10%, which is less than the average mortality rate for severely ill patients with COVID-19.
- Almost half (10 of the 21) of published findings were based on expanded access use of RMCTs for COVID patients
- Only 3 of the 21 published findings were based on randomized, controlled trials, and therefore–as noted above–the conclusions that can be drawn from published findings to date are limited.
Updated as of December 29, 2020